The World Health Organization (WHO) registered the inert SARS-Cov2 vaccine developed at the Kazakh Research Institute for Biological Safety Problems to the shortlist of COVID-19 candidate vaccines in preclinical evaluation on May 15.

Photo credit: inform.kz.
The developers created it on the basis of a virus strain isolated from patients in Kazakhstan. The vaccine will undergo a series of preclinical and clinical trials.
“This is a significant contribution of Kazakh science to ensuring the biological safety of our state, as well as evidence of the country’s strong scientific potential. The staff of the institute have once again shown their leading role in the development of new types of vaccines against dangerous diseases,” Kazakh Minister of Education and Science Askhat Aymagambetov congratulated the research institute in a telegram, quoted by inform.kz.
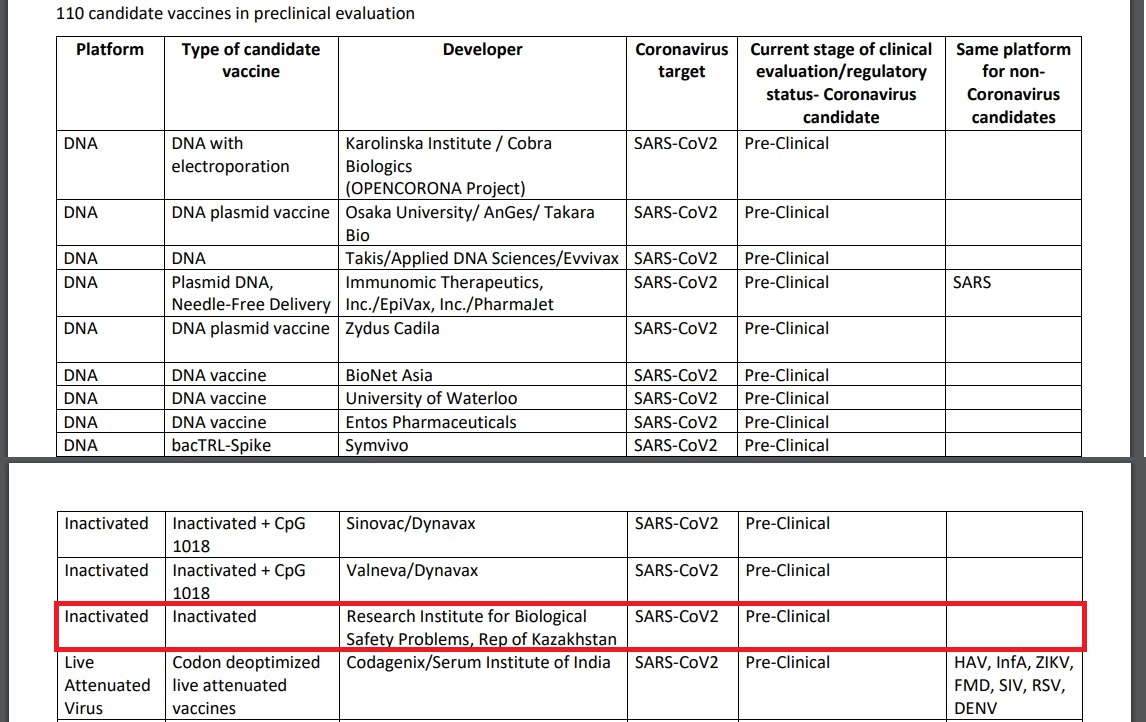
A screenshot from the WHO’s draft landscape document of COVID-19 candidate vaccines as of May 15.
The Kazakh Research Institute of Biosafety Problems is part of the Science Committee of the Ministry of Education and Science. It is Kazakhstan’s only state organization that decides on the development of domestic technologies for the manufacture, testing and registration of medical immunobiological medications at the Kazakh Ministry of Health.
The institute has a storied history of more than 60 years. It has been engaged in the development of threat detection tools and methods, biological safety assessment and the manufacture of diagnostic and preventive drugs, including those against especially dangerous viral diseases found in humans, animals and birds.
It has developed and introduced into production 67 types of vaccines, 15 test systems, and more than 30 biological products. The institute’s first registered vaccines are influenza A / H5N1 Kazfluvac (bird flu) and A / H1N1Refluvac (swine flu).
The institute has a contract with the WHO for vaccine development and conducts joint research with leading research centers and institutes of the world such as the Federal State Budget Scientific Institution Research Institute of Influenza and the Research Institute of Primatology (Russia), among others.
Kassym-Jomart Tokayev visited the National Center for Biotechnology and instructed the institute to continue work on the development of a COVID-19 vaccine on March 25th.
