ASTANA – FutureMed Ventures KZ, a Kazakh-American venture fund, seeks to foster Kazakhstan’s emerging role as a destination for clinical trials and bring innovative technologies to the country, said Almas Kenessary, the fund’s managing partner, in an interview with The Astana Times. He discussed the fund’s goals, a brain tissue regeneration technology it invests in, and why Kazakhstan is poised to become a reliable base for medical innovation.

Almas Kenessary previously served as the head of the Department of Science at the Kazakh Ministry of Health. He is also one of the authors of the legislation on conducting biomedical experiments, preclinical (non-clinical) and clinical studies. Photo credit: Kenessary’s personal archive
Delaware-based venture fund
The fund was established in January in Delaware, the United States, and is operationally based in Kazakhstan. It capitalizes on its “unique de-risking concept.”
“In terms of strategic goals, we are a biotech, medtech-focused venture capital firm. Our unique de-risking concept significantly hastens not only Kazakh scientific progress but also global,” said Kenessary.
The fund targets promising developments in the biotech and medtech sectors. It attracts biotech and medtech companies from across the globe, particularly from the American market. Kenessary stressed one of the standout features of the firm’s approach is the ability to conduct early-stage clinical trials in Kazakhstan at a fraction of the usual cost and in a significantly shorter time frame.
Kenessary also added the fund focuses on identifying technologies in phase 2 clinical trials, where efficacy is the primary endpoint.
Stroke recovery technology
Kenessary revealed that the fund has invested in technology focusing on brain tissue regeneration after severe ischemic strokes. It completed phase 1 trials in the United States and is slated to conduct phase 2 trials in Kazakhstan in December.
“At the moment, there are no approved technologies for brain tissue regeneration after severe ischemic stroke,” said Kenessary
He highlighted the prevalence of ischemic stroke, which accounts for the majority of all stroke cases. “There are two types of stroke: hemorrhagic and ischemic. Ischemic strokes are from 70% to 90% of all strokes, depending on the sources. It is a huge burden not only for the U.S. population but globally,” said Kenessary.
According to him, in the United States, the financial burden of post-stroke rehabilitation is staggering, with insurance companies spending anywhere from $40,000 to $120,000 per patient on average.
“We are planning to conduct phase 2b trials here in Kazakhstan. The sample size is 120 patients. That is a placebo-controlled, double-blinded trial. If we show high efficacy, we will submit for conditional approval to the FDA [Food and Drug Administration] agency, which means that the U.S. population shall have access to such revolutionary technology,” said Kenessary.
The preliminary calculations of the cost of this single-injection therapy are around $15,000 to $20,000.
“If a patient shows no significant positive dynamics within the first six months after a stroke, the chances of a patient to rehabilitate totally are super low,” he explained. “There are cases after severe ischemic stroke with left- or right-sided impairments. That means people are not able to brush their teeth or eat themselves. We are giving such patients chances to rehabilitate and become an active cell of the society, once again.”
Estimated timeline
The first injections are scheduled for December. The studies last 12 months.
After six months, the efficacy of the treatment is assessed, and by the end of the 12-month period, the team evaluates whether there are any side effects.
Once the study is complete, they plan to submit the application to the Californian FDA.
Because this is a phase 2 trial, Kenessary said they aim for conditional approval.
“The final BLA [Biologics License Application] or NDA [New Drug Application] is usually granted by the FDA after a phase 3 trial,” he added.
The trial is expected to be completed in 2025. Kenessary said they hope to receive the conditional approval by the end of 2026.
“2027 will be the year of the first distribution of the drug among the U.S. population,” he said.
Clinical trials in Kazakhstan
Kazakhstan might not be the first country that comes to mind when thinking about cutting-edge clinical trials, but Kenessary sees it differently. Although Kazakhstan might not match the healthcare standards of the United States or Japan, it certainly holds its own, particularly in specific areas such as cardiovascular surgery.
He cited the Global Health Security Index, which ranks Kazakhstan 55th out of 195 countries. Developed by the Nuclear Threat Initiative (NTI), the Johns Hopkins Center for Health Security, and Economist Impact, the ranking assesses countries’ capacities to prevent, detect, and respond to infectious disease threats, including pandemics.
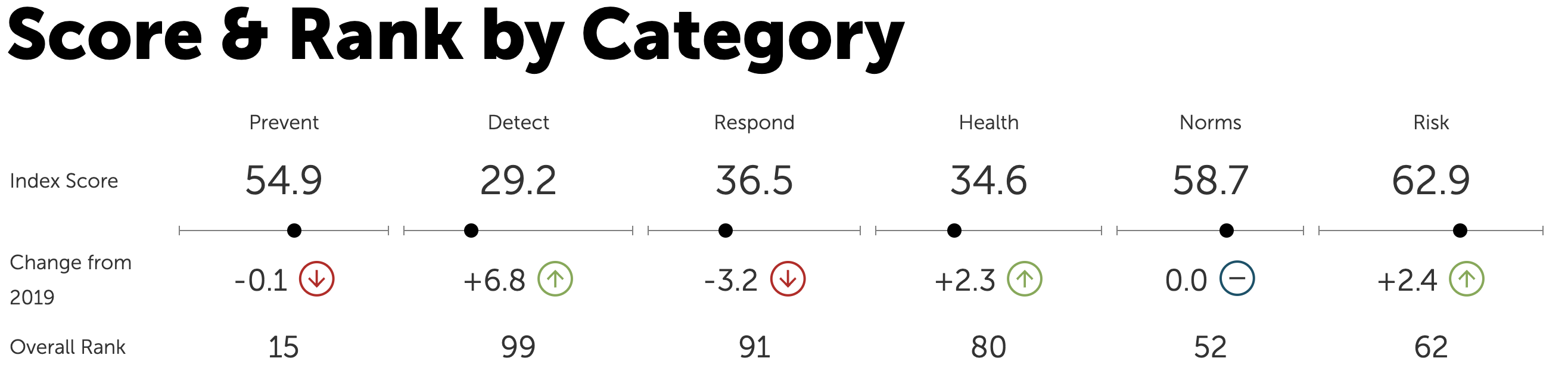
The GHS Index assesses countries’ health security and capabilities across six categories and 37 indicators. This is Kazakhstan’s ranking.
Kenessary is sure Kazakhstan can easily compete with developed countries in some sectors, such as cardiovascular surgery. He said that when international medical experts, such as heads of clinics and vascular surgeons from the United States, visit Kazakhstan, they are often surprised by the capabilities of local facilities.
“When we take them to our cardiovascular surgery clinics, they say, even 10% of clinical facilities in the United States are not capable of conducting such sophisticated operations. Kazakhstan conducts transplantation of artificial hearts and ventricular. We were the first country in the world to transplant donor heart after artificial heart,” said Kenessary.
In addition, many of Kazakhstan’s clinics have achieved Joint Commission International (JCI) accreditation, the gold standard for healthcare service quality in the United States.
One key advantage Kazakhstan offers to biotech and medtech companies is the significantly lower cost and faster timeline for conducting clinical trials.
“One of our portfolio companies developed a bioresorbable stent for peripheral arteries. Initially, they were seeking $15 million to conduct their trial in the United States. I told them, ‘First, we conduct a super cheap and super fast trial in Kazakhstan.’ You will prove the efficacy of the stent. If everything goes well, we will repeat the trial in the United States,” he said.
According to him, the entire trial, with 60 operations, will cost $378,000. Such substantial cost reduction is possible because the cost of performing stent implantation in Kazakhstan is a fraction of that in the United States, even when using the same high-quality stents from major manufacturers.
The same operation that costs $15,000 to $110,000 in the United States costs less than $3,000 in Kazakhstan.
Overall, Kenessary stressed the cost-effectiveness of clinical trials in Kazakhstan.
“On average, a phase 2 trial costs around $20 million in the United States. For the same amount of money, we can test up to 30 technologies here in Kazakhstan,” he said.
“The price of the drug in the United States always includes all the expenses of unsuccessful trials,” he added. “Using international clinical sites significantly decreases the cost of the trials, thus decreasing the price of the drugs in the end.”
Speed is another critical factor. While the trial itself cannot be rushed due to adherence to FDA-approved protocols, the bureaucratic and operational procedures are streamlined.
“Today, we are capable of receiving permission to conduct clinical trials within two to four months [in Kazakhstan]. In the United States, to receive an IND [Investigational New Drug] designation, it usually takes nine to 12 months on average,” he explained.
Kazakhstan’s centralized healthcare system also provides a significant advantage in patient recruitment, a process that can be a bottleneck in clinical trials.
Kenessary noted the plan is to recruit 120 patients for phase 2b trials for ischemic stroke technology within three months. This speed is possible because the firm can access a centralized database of post-stroke ischemic patients and can reach out directly to their physicians.
“The company’s CEO always tells me if we were to conduct the trials back in the United States, it would take two to three years,” Kenessary added.
Clinical trials market in Kazakhstan
Kenessary noted a transformation of the clinical trials market in Kazakhstan in recent years.
“Some three to five years ago, due to the fact the clinical trials market was severely undeveloped, the society considered clinical trials to be something hilarious or negatively notorious,” he said.
However, he noted the narrative is changing, especially among doctors.
According to him, pharmacogenetics—the study of how genetic differences among individuals influence their response to drugs—is a key reason why clinical trials are so critical.
The full interview is available on The Astana Times’ YouTube channel.
